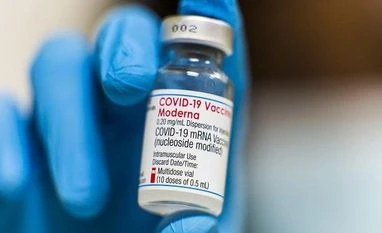
Covid-19 vaccine for younger kids showed strong results, says Moderna
Health officials are aiming to get immunizations to younger children before the holiday season, when travel and indoor activity can bring an increased risk of infection
)
Explore Business Standard
Health officials are aiming to get immunizations to younger children before the holiday season, when travel and indoor activity can bring an increased risk of infection
)
‘Safe for kids’
- Biotech preparing to ask US, EU regulators for clearance
- Children ages 6 through 11 developed strong immune response
- Moderna said it plans to submit the data to the Food and Drug Administration, the European Medicines Agency and other global regulators in the near term
Already subscribed? Log in
Subscribe to read the full story →

3 Months
₹300/Month
1 Year
₹225/Month
2 Years
₹162/Month
Renews automatically, cancel anytime

Over 30 premium stories daily, handpicked by our editors



News, Games, Cooking, Audio, Wirecutter & The Athletic

Digital replica of our daily newspaper — with options to read, save, and share



Insights on markets, finance, politics, tech, and more delivered to your inbox

In-depth market analysis & insights with access to The Smart Investor



Repository of articles and publications dating back to 1997

Uninterrupted reading experience with no advertisements



Access Business Standard across devices — mobile, tablet, or PC, via web or app
First Published: Oct 26 2021 | 2:03 AM IST