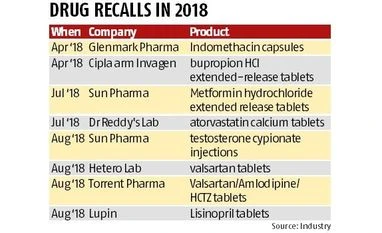
The impurity detected in the API is N-nitrosodimethylamine (NDMA), which is a substance that occurs naturally in certain foods, drinking water, air pollution, industrial processes, has been classified as a probable human carcinogen, according to International Agency for Research on Cancer classification. Earlier this month, Hetero Laboratories' valsartan products, too, were added in the recall list for containing trace amounts of NDMA by the US Food and Drug Administration (USFDA).
Analysts say that this essentially means that the companies will have to change their source for the API, take fresh approvals for the product containing the new API and then relaunch it in the US market. Torrent Pharma has already stopped sourcing valsartan from Zhejiang Huahai Pharmaceuticals.
)












