Expert panel likely to take call on Sputnik V vaccine at key meet today
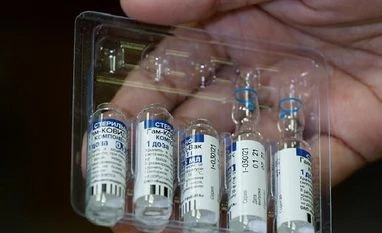
Russia registered Sputnik V for public use in August, the first country to do so
)
Explore Business Standard
Russia registered Sputnik V for public use in August, the first country to do so
)
The Subject Expert Committee (SEC) is likely to meet today to take up Sputnik V application for Emergency Use Authorisation (EUA) in India, said sources.
Russia's Sputnik V vaccine did not get the EUA on Thursday as the SEC, following the meeting, asked for more information.
According to the sources, 'The SEC has asked for more information in context with the approval of the Russian vaccine."
The committee, set up by the government, looked into Dr Reddy's application seeking approval for the vaccine to be used in India. The Russian vaccine has an effectiveness of 91.6 per cent. Dr Reddy's Lab has partnered with the Russia Direct Investment Fund (RDIF) to bring the Sputnik V vaccine to India and other countries.
Russia registered Sputnik V for public use in August, the first country to do so, though the approval came before the start of the large-scale trial in September.
Top government sources told ANI that by end of the third quarter of this year, India will be getting vaccines from five additional manufacturers. India currently manufactures Covishield and Covaxin.
When a top-level source was asked when the vaccines will be available for use, he said, "Sputnik is expected to be available latest by June, if all goes well Johnson and Johnson ( Bio E) will be available by August, Cadilla Zydus will also be available by August, Novavex (Serum) by September and Nasal Vaccine (Bharat) by October."
The Government is making all efforts to accelerate the progress without cutting any corners in research, development, and clinical trial stages, the source added.
India is currently facing the second wave of COVID-19 pandemic with the number of new cases increasing each day.
First Published: Apr 12 2021 | 2:49 PM IST