Cadila: Concerns over US, India growth remain
With little clarity on timing of Moraiya plant clearance by FDA, gains from product launches might get delayed
Ujjval Jauhari New Delhi Following Dr Reddy’s footsteps, Cadila Healthcare, too, has disappointed with its March quarter performance. Both companies are facing US Food and Drug Administration (FDA)-related issues, resulting in lower approval rates for product launches and, hence, softer growth.
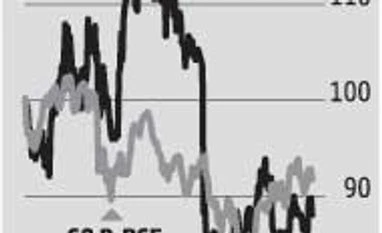
Cadila’s Moraiya plant, which accounts for about 60 per cent of its US revenue, has been under the FDA scanner since 2014. Anti-malarial drug Hydrochloroquine, which had been driving its US growth so far, is seeing competition. Prices of this drug are down 55 per cent from the peak of May 2015, says Nomura. US sales, expected to decline on a sequential basis, are still lower than estimates. At Rs 970 crore, these are down 10.3 per cent from the December quarter. Consequently, total revenues at Rs 2,376 crore (up 5.7 per cent, year-on-year), operating earnings at Rs 508 crore and net profit of Rs 389 crore were lower than the Bloomberg consensus estimate of Rs 2,502 crore, Rs 579 crore, and Rs 396 crore, respectively. The stock, thus, corrected about two per cent to close at Rs 327 on Friday.
For now, Cadila remains focused on the US market. It expects about 20 new product approvals from various other facilities in FY17, and is also taking site transfers to ensure sales momentum. FDA allows companies to produce products approved by it for export to the US at another approved site if the original site is facing regulatory issues. Site transfers for 12 existing products have been taken and more are planned. Overall, 166 products are pending FDA approvals. The major approval the Street is watching is for Asacol HD, to treat ulcerative colitis. This was to be launched on exclusivity in November but has not received approvals as the product was filed from Moraiya.
Given the uncertainties, the current expectation of Asacol launch in July might also not materialise. While company has replied to the FDA on remediation measures, it is likely to give one more update this month-end and invite the agency for audit. Also, pricing pressure for its malaria drug will remain for some more quarters, Cadila’s near-term US growth will continue to be under pressure. Concerns over domestic growth also prevail, looking at the new product pricing caps, ban on fixed dose combinations and soft demand. In April, the India pharma market grew at a subdued 3.5 per cent but Cadila is estimated to have grown by only 1.5 per cent or two per cent.
)
)