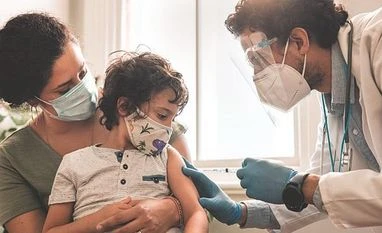
Explained: What it takes to run child trials for a Covid-19 vaccine
JAB AT HAND: From encouraging people to volunteer to screening kids and winning their confidence
)
premium
If the staff senses hesitancy in the child, it does not enrol the kid for trials even if parents have consented
7 min read Last Updated : Nov 08 2021 | 6:04 AM IST
Anjana Tewari (name changed) is unhappy that she got late by a day for registering her two-and-a-half-year-old daughter for paediatric vaccine trials at the All India Institute of Medical Sciences (AIIMS) Patna. She says she did have apprehensions about possible adverse reactions during the trial, “but is there no risk in keeping my daughter unvaccinated? My husband and I are both fully vaccinated with Covaxin and we worried about our daughter not having the same level of protection.”
AIIMS Patna was among a dozen sites nationwide that were earmarked for phase 2 and 3 paediatric trials of Covaxin. And among those who took a leap of faith was an associate professor at the hospital, who chose to register both her sons, aged seven and 14, for the trials. In December 2020, this doctor had also volunteered for phase 3 adult trials of Covaxin, the Covid-19 vaccine domestically manufactured by Bharat Biotech.
The first vaccine to get emergency use authorisation (EUA) for 12- to 18-year-olds in India is the needle-free Zycov-D from Zydus Cadila. This is a three-dose vaccine, to be given on day zero, day 28 and day 56. Bharat Biotech’s Covaxin, already in use for adults, is awaiting EUA. And paediatric trials are on for the third vaccine, Covovax, which will be manufactured by the Serum Institute of India in partnership with US-based Novavax Inc.
Behind each of these vaccines are hundreds of parents who had the courage and conviction to come forth with their
children for the trial of a vaccine, which was, until then, untried in that age group. India has a large paediatric population — estimated at anywhere between 42 and 44 per cent, hence the urgent need for a children’s vaccine, and for volunteers.
At AIIMS Patna, trials were conducted in three batches, says Sanjeev Kumar, head of Cardio Thoracic Surgery and the nodal officer for Covid-19: first, for 12-18-year-olds, then 6-12 and lastly for 2-6. Older children were given the doses first because the chance of any adverse reaction in them is lesser. Adults have “more cardiac reserve than kids; they have better developed organs, so any adverse reaction can be dealt with better,” Kumar explains.
Vax trial onboarding
In the 12-18 age group, AIIMS Patna screened 185 children but only 90 were ultimately administered both doses of Covaxin. The rejections were due to the exclusion criteria, which included fever or existing acute illness, severe malnourishment or previous exposure to Covid-19. Kumar said nearly every second child of the 185 tested was found sero positive (had Covid-19 antibodies) and had to be turned back.
“We used print media and television for appeals to the public to come forward for trials, told them about benefits of vaccines and also pointed out that by allowing their children to participate in these trials, parents would be creating history,” says Kumar. “We also assured parents that only 50 per cent of the participants would be getting the vaccine; the remaining 50 per cent would be given a placebo. And also pointed out that those children who get vaccinated during the trials would become safe from Covid-19 much before other children do.”
The doctor from the hospital who registered her children for the trial was also actively involved in a month-long campaign to enrol kids, and she coordinated with Delhi Public School, Patna and Foundation School, Buxar. These two, and other local schools, were roped in for educating parents about the benefits of the vaccine and encouraging them to bring their children for trials. She says that in Buxar, she shared her own experience of participating in the adult trial and to convince parents, she emphasised that the vaccine shot produced “minimal side effects”. Teachers, senior students and principals of different schools in the state were spoken to, and she says that apart from a few, most children who eventually participated in the trials were from non-medical families.
The trial was on for two months for the 12-18-year-olds. Some kids developed fever and some had pain at the injection site. But none faced any severe allergic reaction and no participant needed hospitalisation, Kumar says. Then the hospital turned to the 6-12-year group. Here, nearly 60 kids participated in the trials; and for the 2-6 age bracket, 50 were administered the vaccine. In neither of these two age groups, too, were there any adverse events, says Kumar.
Well aware of how kids feel about needles, the hospitals tried to take some sting out of the exercise. For smaller kids, the vaccination site was made child friendly, and decorated with colourful posters, toys and balloons. Children were allowed to remain in their parents’ lap when the injection was being administered. And parents were offered up to Rs 800 as conveyance reimbursement for bringing their kids for the vaccine trial.
A matter of trust
Pune’s KEM Hospital is one of the 10 designated sites for paediatric trials for Covovax. Ashish Bavdekar, paediatric consultant with the hospital, says KEM did not encounter any hesitancy in parents, and initial recruitments for paediatric trials happened smoothly.
KEM Hospital has been conducting large vaccine trials for the last 20 years and has a full clinical trial unit. It was also the site for adult trials for Sputnik and Covishield vaccines, besides adult trials for Covovax.
Bavdekar says parents are generally not hesitant because the vaccine maker is well known and trusted; there is enough data already available to assure them of nearly 95 per cent protection, and paediatric trials done in other countries have been safe. “Most adults are already vaccinated and hence not keen to take part in studies for new vaccines themselves, but they are keen to get their kids vaccinated,” he says.
However, if the staff senses hesitancy in the child, it does not enrol the kid for trials even if parents have consented because multiple visits and jabs are needed for the trial and in the follow-up period.
At PGIMER Chandigarh, too, paediatric trials for Covovax are starting. Madhu Gupta, the principal investigator, says the first batch will comprise kids between the ages of 7 and 12; and 2-7-year-olds will follow.
All the approved trial sites follow competitive recruitment. So each site competes for the number of children recruited in each age group from the approved sample size. PGIMER has made registration easily accessible by putting up a link on its website.
The trials are sponsored by SII. The notice makes it clear that participation is “purely on a voluntary basis” and that children over 12 can self-register.
Gupta says no incentives — not even toys — are on offer, and only the travel spend of parents will be reimbursed. Only word-of-mouth publicity is being done for the paediatric trials but the hospital may consider more publicity programmes if the need arises. The target is to recruit 150-200 children between 2-17 years and ultimately conduct trials on 100. “Generally people trust PGIMER, so that trust will help in encouraging parents to bring their kids for trials,” Gupta says.
Vaccines and trust clearly go a long way.