India Coronavirus Dispatch: Syringe makers ramp up production capabilities
Scores of Keralites return home from abroad, why politicising health is a bad idea, how Mumbai plans to inoculate its frontline staff-news relevant to India's fight against Covid-19
)
premium
Both Serum Institute of India (SII) and Bharat Biotech vaccines have been approved for emergency and restricted use in the country
4 min read Last Updated : Jan 08 2021 | 2:17 PM IST
Syringe makers ramp up production capabilities
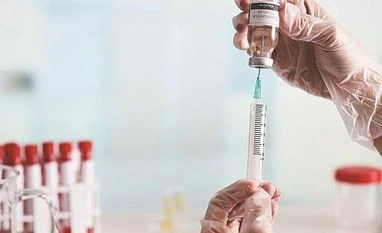
As India inches closer towards kicking off the Covid-19 vaccination drive, syringe makers have been hard at work to ramp up production capabilities, according to a report in The Quint.
India's national drug regulator announced on January 3 that the Covid-19 vaccines of both Serum Institute of India (SII) and Bharat Biotech have been approved for emergency and restricted use in the country. The country is planning to begin the vaccination drive on January 13.
According to early estimates, about 1.80 billion syringes will be required just to inoculate at least 6 in 10 people in India. Syringe makers may need to rethink their "current process" if the Centre is eyeing to inoculate a significant portion of the population by September, said Sandeep Bhandari, vice president at Rajasthan-based syringe manufacturer Iscon Surgicals. The syringe makers hope the Centre can provide them with assistance and guidance in working together with the vaccine manufacturers. They also seek more information from the central government on the demand for the various types of syringes, the Quint report said. Read more here
Over 550,000 migrants from Kerala return from abroad after losing their jobs
As the pandemic-induced lockdowns and other curbs ravaged the global economy, over 550,000 Keralites working abroad are out of the workforce and have returned home, according to a report in The Indian Express.
About 843,000 people returned to Kerala from abroad between the first week of May 2020 and January 4 this year. Among those, 552,000 people said they had lost their jobs, according to figures compiled by the Department of Non-Resident Keralites Affairs. The figures point to a continuing impact on the Kerala economy as the job losses will likely hit remittances from abroad, mainly from West Asia. Incidentally, as expats return to Kerala, NRI deposits in the state’s banks have been rising, the report said. Read more here
OPINION: Why politicising health is a bad idea
The time is ripe to 'politicise' the need for a robust healthcare system in every Indian state, and not the time for politicking over Covid-19 vaccines, said an opinion piece in The Quint by Chandrakant Lahariya, a medical doctor and public policy and health systems expert.
Health has been a low priority for political parties in India, at least historically. Election manifesto promises are rarely followed through on. The attention health receives during election campaigns does not translate into actual change on the ground, the opinion piece said.
Investments and initiatives by the governments and political parties have been a case of "too little too late". They are mostly notional in nature with minor tweaks to ongoing health programs. However, with the Covid-19 pandemic, the development and distribution of vaccines have become part of the mainstream discourse already. This year, hopefully, political leaders and the citizenry will not forget about the challenges facing India's public health systems. Ignoring to continue the discourse will be a colossal failure, the piece said. Read more here
Serum Institute Vs Bharat Biotech: Science must lead the way
In the fight to contain the defiant novel coronavirus and inoculate hundreds of millions of people, we should not be distracted by the controversies around unclear regulatory rulings or crossfire between vaccine manufacturers, said an opinion piece in The Quint by K Srinath Reddy, president of the Public Health Foundation of India.
After the DCGI approved SII's Covishield, and Bharat Biotech’s Covaxin for restricted emergency use in the country, a group of experts questioned the approval process for Covaxin, triggering a war of words, Business Standard reported on January 6. Later, the chiefs of SII and Bharat Biotech called a truce and pledged to work together for a “smooth roll-out” of Covid-19 vaccines.
The moral of the story is that science must lead the way, the opinion piece said. India's health regulators must ensure to share critical findings with the wider scientific community and the general public, including the shortcomings. If the regulator can then make a compelling argument for a decision based on the evidence available, there would be a willingness to accept the shortcomings in view of the larger interest, the opinion piece said. Read more here
How Mumbai will inoculate its frontline workers
Mumbai, a Covid-19 hotspot, is set to begin inoculating about 126,000 health workers as and when the vaccines become available. This report in The Indian Express explains how the beneficiaries will be contacted by the Brihanmumbai Municipal Corporation (BMC), the strategy to tackle vaccine hesitancy for the first dose or the second dose, and where the vaccines will be stored. Read more here