Moderna to charge govts $25-$37 for Covid-19 vaccine: CEO tells paper
Moderna has said its experimental vaccine is 94.5% effective in preventing Covid-19, based on interim data from a late-stage clinical trial.
)
premium
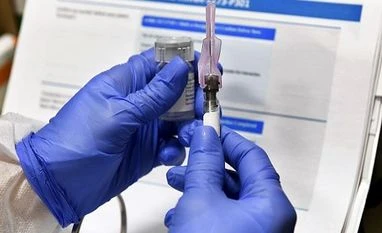
In this July 27, 2020, file photo, a nurse prepares a shot that is part of a possible Covid-19 vaccine, developed by the National Institutes of Health and Moderna Inc., in Binghamton, N.Y. (AP Photo/Hans Pennink, File)
Moderna will charge governments between $25 and $37 per dose of its Covid-19 vaccine candidate, depending on the amount ordered, Chief Executive Stephane Bancel told German weekly 'Welt am Sonntag' (WamS).

