Pharma majors plan $2-billion investments to tap new business
India's drug makers spent $1.4 billion in the previous financial on R&D
)
premium
Data
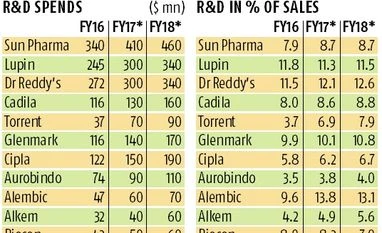
Indian pharma companies’ annual spending on research and development (R&D) might cross $2 billion by 2017-18.
Companies such as Sun Pharma, Lupin and Dr Reddy’s have increased their spending to develop generic versions of complex drugs that are going off-patent in the world’s largest drug market, the US.
India’s drug makers spent $1.4 billion in the previous financial on R&D and are expected to spend about $1.7 billion in the current financial, according to industry estimates.
The rising R&D spend is seen as Indian companies’ attempt to protect themselves from the price erosion that plain generic drugs are facing in the US due to increasing competition and regulatory pressure. Complex generic drugs take longer time and cost more to develop, but provide protection from competition and price erosion.
“Unlike most approved generic drugs, complex generics are difficult to analyse and, therefore, difficult to reproduce in comparison to plain generics which leads to less competition,” said a company spokesperson at Mumbai-based Glenmark, which entered complex products’ market early on by launching dermatology and oral contraceptive products in the US. According to analysts’ estimates, the company was expected to spend $160 million on R&D in FY 18, up from $116 million in FY16.
Companies such as Sun Pharma, Lupin and Dr Reddy’s have increased their spending to develop generic versions of complex drugs that are going off-patent in the world’s largest drug market, the US.
India’s drug makers spent $1.4 billion in the previous financial on R&D and are expected to spend about $1.7 billion in the current financial, according to industry estimates.
The rising R&D spend is seen as Indian companies’ attempt to protect themselves from the price erosion that plain generic drugs are facing in the US due to increasing competition and regulatory pressure. Complex generic drugs take longer time and cost more to develop, but provide protection from competition and price erosion.
“Unlike most approved generic drugs, complex generics are difficult to analyse and, therefore, difficult to reproduce in comparison to plain generics which leads to less competition,” said a company spokesperson at Mumbai-based Glenmark, which entered complex products’ market early on by launching dermatology and oral contraceptive products in the US. According to analysts’ estimates, the company was expected to spend $160 million on R&D in FY 18, up from $116 million in FY16.

