Compulsory licensing could be big business for drug makers
)
With over 500,000 people dying of cancer every year in India, the argument over compulsory licensing seems to be headed in one direction - in favour of those who can produce inexpensive life-saving drugs. Thus, Natco Pharma has secured the first ever compulsory licence to make the generic version of Bayer's patent-protected anti-cancer drug, Nexavar. Mumbai-based BDR Pharmaceuticals has applied for a similar licence to make Bristol-Myers Squibb's anti-cancer drug Sprycel. And the government is evaluating handing out compulsory licences for three other drugs used in the treatment of cancer.
Local manufacturers, which have strong reverse-engineering capabilities, see huge business possibilities here. For multinational corporations, faced with a drying pipeline of patented products, this represents a return to the pre-2005 days when India recognised only process patents and not product patents, and therefore Indian companies made every possible medicine in the world with impunity. This will stifle research and hinder innovation, they have argued. The Rs 67,000-crore Indian pharmaceutical market, which has seen sharp divisions in the swadeshi and videshi camps, is getting polarised over this issue too. While domestic companies such as Cipla, Natco and Lupin, which are proficient in developing low-cost drugs, are increasingly eyeing opportunities to develop generic versions of expensive patented products, multinationals such as Bristol-Myers Squib, Roche, Novartis and Pfizer are evaluating strategies to protect their patents and profits.
A compulsory licence is a provision under the Indian Patent Act which allows the government to mandate a generic drug maker to produce inexpensive medicine in public interest even as a patent on the product is valid. The provision provides a rare flexibility on patent protection included in the World Trade Organisation's agreement on intellectual property. Several developing and developed countries, including Italy and Thailand, have more drugs under compulsory licences than India.
New opportunity
The spree of recent actions surrounding compulsory licences against significant patented medicines in the country, according to industry experts, has opened up a new window of opportunities for domestic pharmaceutical companies. "The movement on compulsory licensing is certainly positive for domestic companies. Generic companies are now going to single out therapies which are novel, high-priced and have high value. The first step is to identify such products and then seek compulsory licence against them mainly on the pretext of pricing," says Aashish Mehra, partner and managing director, Strategic Decisions Group, a US-based strategy consulting firm. According to Mehra, domestic companies exploring this opportunity are mostly eyeing niche therapy areas with unmet needs such as oncology and the retroviral segment.
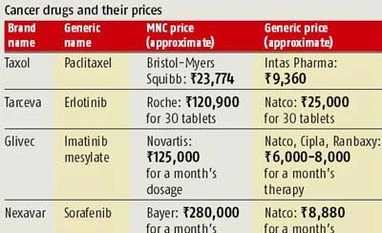
Last year, the first instance of compulsory licensing came in with the Patent Controller granting permission to Natco for manufacturing generic version of Bayer's patent-protected cancer drug Nexavar. Natco was allowed to sell its version on the condition that a month's dose would not cost more than Rs 8,880, against Rs 280,000 charged by the German drug maker. While Bayer challenged the regulator's decision, the Intellectual Property Appellate Board has upheld the compulsory licence granted to Natco. "With the precedent been set in the case of Nexavar, there are likely to be other products in the fray," says Utkarsh Palnitkar, partner and national head (life sciences practice), KPMG.
"The decision on Nexavar is significant because it, in a way, establishes India's approach towards making medicines affordable. This is bound to encourage more companies to opt for such routes by developing cheaper versions of important patented drugs," another pharma sector analyst says. More recently, Mumbai-based drug firm BDR Pharmaceuticals has applied for a compulsory licence under the same section as Natco did to market the generic version of Bristol-Myers Squibb's anti-cancer drug Sprycel. BDR Pharma has promised to offer the product at a price that is over 95 per cent cheaper for a month's treatment. "As against Bristol-Myers Squibb's monthly treatment cost of Rs 165,680, BDR will offer the product at Rs 8,100 per month, which works out to around 95.2 per cent cheaper," BDR Pharma says in a statement.
The move towards invoking compulsory licence to make more essential drugs available is not just limited to domestic companies. Lately, even the government has shown reasonable interest in the issue. It is evident from the fact that a panel set up by the central government, under the health ministry, to evaluate the possibility of granting more compulsory licences in the country, has recommended allowing manufacture of low-cost versions of three patented cancer medicines under the 'compulsory licensing' clause of the Indian Patent Law. The drugs in question are Herceptin (Roche), Ixempra and Sprycel (both from Bristol-Myers Squibb). Many in the industry as well as health activists and patient groups expect the government to invoke a compulsory licence in these three cases, similar to that in the case of Nexavar, since the monthly doses of these medicines are also in the Rs 150,000-250,000 range.
"I think as far as the government is concerned, clearly it has to ensure access, availability and affordability. Compulsory licensing is one of the measures by which this can be achieved. Is this a sustainable measure? Only time will tell," says Palnitkar. K M Gopakumar of Third World Network, a non-profit organisation of institutions working for development, says, "The grounds on which compulsory licence has been granted to Natco is a clear signal that other generic drug makers can also apply for other expensive patented drugs."
Room for more
Natco has gained compulsory licence for Nexavar mainly on three grounds under section 84 of the Indian Patent Act. The first is that the patented drug is allegedly not available at a reasonably affordable price. Two, the reasonable requirement of public with respect to the patented invention has allegedly not been satisfied. Three, the patent on the product has allegedly not worked in India as Bayer is importing the medicine and not manufacturing here, Gopakumar explains. However, there are also other provisions in the Indian patent law which allows issuance of compulsory licences. The law has a total of four provisions through which compulsory licences can be issued. For instance, the government is considering invoking section 92 for the three drugs - Herceptin, Ixempra and Sprycel. This would mean that if granted, generic drug makers can directly apply to the patent controller for obtaining a licence, instead of first seeking permission of the innovator company as happened in the case of Nexavar.
However, for some companies, the route may not be that easy. Many of the top Indian drug firms such as Dr Reddy's, Torrent Pharma and Aurobindo Pharma have partnership agreement with multinational drug firms on other areas and invoking compulsory licence could cause a possible harm to such relationships, say experts. "While compulsory licensing could prove to be an opportunity for some companies, it is not an easy path to follow and certainly not one that can be emulated by companies cutting across the sector," says Palnitkar. Experts also view that if pursued actively, compulsory licensing is bound to trigger litigations going forward. "The litigation risk is high and legal costs could be fairly steep, something that many companies can ill afford," says Palnitkar.
However, Gopakumar disagrees. According to him, litigation costs in India are low and would work in favour of companies that want to explore this opportunity. Indian companies are also likely to explore this opportunity more because they have good chemistry skills coupled with low manufacturing cost here, he adds. Pre-empting the move of domestic companies, some multinational companies are trying to introduce "India pricing" for some of their products. These products have stacked up impressive sales since such pricing, says Palnitkar. "I think it will be imperative to be very cognisant of price sensitivities. Such companies will have to explore ways and means of engaging with the government, public bodies and civil society at large to ensure that reasonable profit is not perceived as profiteering," he adds.
Experts, however, fear that introduction of new products by multinational companies could be impacted because of such revolutionary moves and, therefore, there is a need to distinguish between consumers' ability to pay and to ensure that innovators derive a fair return.
More From This Section
Don't miss the most important news and views of the day. Get them on our Telegram channel
First Published: Apr 04 2013 | 12:10 AM IST
