Moderna, Pfizer Covid-19 vaccines look strong: Here's how they stack up
The data will likely increase confidence that more vaccines will work and that the world may soon find a way to get the coronavirus under control
)
premium
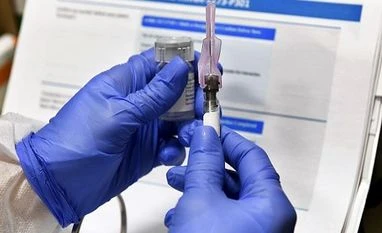
In this July 27, 2020, file photo, a nurse prepares a shot that is part of a possible Covid-19 vaccine, developed by the National Institutes of Health and Moderna Inc., in Binghamton, N.Y. (AP Photo/Hans Pennink, File)
The first two Covid-19 vaccines out of the gate have now delivered positive news in the quest to end the pandemic. The encouraging late-stage trial results from Pfizer Inc. and Moderna Inc. set a high bar for rivals such as AstraZeneca Plc that are expected to follow soon with their own pivotal reports.
Topics : Coronavirus Coronavirus Vaccine Pfizer

