Wockhardt: Too early to rejoice
While the listing of its subsidiary will rub off positively on valuations, resolving of US FDA issues is crucial and not expected soon
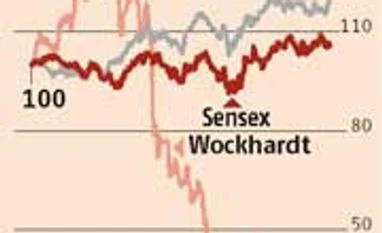)
To investors’ delight, the stock of Wockhardt, which had plunged from Rs 1,900 levels in May to a 52-week intra-day low of Rs 336.60 this Monday, jumped 10 per cent on the bourses to close at Rs 417 on Thursday.
The gains came after its announcement on allocation of shares to investors of its international arm, Wockhardt Bio AG, scheduled to be listed on December 19 on the stock exchange at Berne, Switzerland. Wockhardt Bio AG, Switzerland, has allocated 2,348,000 ordinary shares of Swiss Franc (CHF) 1.00 each at a price of CHF5.05 a share in its Initial Public Offering.
With this, Wockhardt has been able to raise about Rs 90 crore with minimal equity dilution of five per cent, while retaining control on the company. Notably, the deal values Wockhardt Bio at Rs 1,800 crore or 43 per cent of the Wockhardt market cap on Wednesday (prior to the news). So, the gains are not surprising and it is possible that investors could see some more down the line.
Though a positive and encouraging development for a company grappling with issues at both the US and British drug regulatory agencies, the amount of reaction has taken most analysts by surprise. Shriram Rathi at Anand Rathi Institutional Equities does not find any reason for such euphoria in the counter. Sarabjit Kaur Nangra, vice-president, research, at Angel Broking, said the good news for the company after a while might have boosted investor sentiment but the event was not significant enough for such a reaction.
The bigger gains would come if the company was able to resolve issues relating to its two factories in India. After getting alerts (warning strictures) for shipments from its Waluj plant and later its Chikalthana plant (both in Maharashtra), the company has lost almost a sixth of its market capitalisation in the past eight to nine months (52-week high of Rs 2,166.05 on March 12).
After its September quarter results, the management stated it was working to resolve these issues at the earliest, to limit the financial impact. However, these are likely to take time. The remedial measures in accordance with the US Food and Drug Administration’s observations, and the costs to be incurred on these, are likely to keep profitability of the company under stress for a few more quarters. Analysts maintain they do not foresee a resolution to the problem in a short span.
During the September quarter, the company’s US business at $87 million declined 26 per cent in dollar terms and 19 per cent in rupee terms. The import alert on the Chikalthana unit came after this; the Waluj alert was earlier. The company’s major product in the US, the extended release version of anti-hypertensive brand Toprol XL was estimated to be a $120 million per annum product. It, too, came under the import alert after the Chikalthana development. Thus, US sales would further get impacted.
Even before this alert and during the month of October, analysts at Macquarie had reduced their target price to Rs 480, at six times the FY15 estimated price to earnings ratio (from the earlier Rs 750, at seven times the FY14 estimated earnings), as they expected the stock to trade at a discount to fair value in the medium term. The stock is likely to remain under further pressure, they’d noted.
The only good news is that in the domestic arena, trade data for November shows sales grew by nine per cent. Still, the company will require some time to fill the void created by the ban on its Rs 180-crore opioid brand, Spasmo Proxyvon.
The consensus target price for the stock, from analysts polled by Bloomberg since November, is Rs 400.
The gains came after its announcement on allocation of shares to investors of its international arm, Wockhardt Bio AG, scheduled to be listed on December 19 on the stock exchange at Berne, Switzerland. Wockhardt Bio AG, Switzerland, has allocated 2,348,000 ordinary shares of Swiss Franc (CHF) 1.00 each at a price of CHF5.05 a share in its Initial Public Offering.
With this, Wockhardt has been able to raise about Rs 90 crore with minimal equity dilution of five per cent, while retaining control on the company. Notably, the deal values Wockhardt Bio at Rs 1,800 crore or 43 per cent of the Wockhardt market cap on Wednesday (prior to the news). So, the gains are not surprising and it is possible that investors could see some more down the line.
The bigger gains would come if the company was able to resolve issues relating to its two factories in India. After getting alerts (warning strictures) for shipments from its Waluj plant and later its Chikalthana plant (both in Maharashtra), the company has lost almost a sixth of its market capitalisation in the past eight to nine months (52-week high of Rs 2,166.05 on March 12).
After its September quarter results, the management stated it was working to resolve these issues at the earliest, to limit the financial impact. However, these are likely to take time. The remedial measures in accordance with the US Food and Drug Administration’s observations, and the costs to be incurred on these, are likely to keep profitability of the company under stress for a few more quarters. Analysts maintain they do not foresee a resolution to the problem in a short span.
During the September quarter, the company’s US business at $87 million declined 26 per cent in dollar terms and 19 per cent in rupee terms. The import alert on the Chikalthana unit came after this; the Waluj alert was earlier. The company’s major product in the US, the extended release version of anti-hypertensive brand Toprol XL was estimated to be a $120 million per annum product. It, too, came under the import alert after the Chikalthana development. Thus, US sales would further get impacted.
Even before this alert and during the month of October, analysts at Macquarie had reduced their target price to Rs 480, at six times the FY15 estimated price to earnings ratio (from the earlier Rs 750, at seven times the FY14 estimated earnings), as they expected the stock to trade at a discount to fair value in the medium term. The stock is likely to remain under further pressure, they’d noted.
The only good news is that in the domestic arena, trade data for November shows sales grew by nine per cent. Still, the company will require some time to fill the void created by the ban on its Rs 180-crore opioid brand, Spasmo Proxyvon.
The consensus target price for the stock, from analysts polled by Bloomberg since November, is Rs 400.
More From This Section
Don't miss the most important news and views of the day. Get them on our Telegram channel
First Published: Dec 19 2013 | 10:46 PM IST
