Wockhardt: Final clearance of Chikalthana unit a few quarters away
Early inspection boosts investors' confidence; analysts expect US supplies to start only in FY17
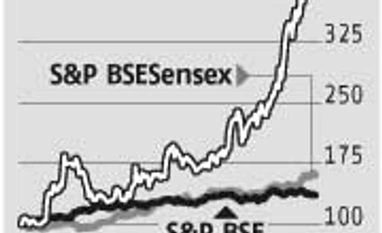)
Investor sentiment in Wockhardt reached its peak on Thursday, as its Chikalthana plant unit L1 is likely to get the US Food and Drugs Administration (FDA) clearance soon. This key facility for Wockhardt was the largest contributor to US sales (around $250 million) before getting import alert in November 2013.
The early inspection at the facility comes as a positive surprise for the Street. Analysts at Macquarie say they have not built opening up of L1 till FY17-end. If the company is able to resolve regulatory issues at this facility by FY16-end, it would be a big booster to FY17 earnings.
Earlier, the company had offered all its facilities for inspection by the USFDA post-implementation of the desired GMP (good manufacturing practices) remediation in October 2014.
These included their new Shendra facility located at MIDC in Aurangabad apart from the Chikalthana and Waluj facilities that have faced the ire of US FDA. With the company stating there were no findings related to data security and control measures implemented by them in the laboratory (L1) at Chikalthana, the prospects of the unit getting faster clearance has increased.
Investor confidence in Wockhardt, however, has been building up for some time after the Chikalthana plant getting approval for supplies to the UK from UK regulatory authority (UKMHRA) in December 2014, with the stock having almost doubled.
The UK revenues that were at £81 million (more that Rs 800 crore) in FY14 had already crossed £109 million (more than Rs 1,000 crore) during first nine months of FY15 and post-clearance, the UK business is to gain more traction. The company’s US-based Morton Grove facility, inspected by FDA earlier, had also not seen any adverse action by FDA (FDA accepted Wockhardt’s responses to their observations) and reports suggest Wockhardt has got clearance for the plant.
If the supplies to the US start sooner than expected, one can expect some earning upgrades.
If the company is able to start supplies after approval of newly expanded Shendra plant early in FY17, analysts at Macquarie arrive at FY17 EPS estimate of Rs 85 and target price of Rs 1,700 (without assuming any contribution from Waluj or Chikalthana).
But, if Chikalthana L1 unit gets approved by US FDA by end-FY16 or early FY17 and Wockhardt is able to recover 40-50 per cent of lost sales from L1 in FY17 (peak sales of $250 million) it could generate $100-125 million in incremental US sales resulting in FY17 EPS of around Rs 125 given the operating leverage that exist. And hence, the Macquarie analysts peg the target price at Rs 2,500.
Thus, while early inspection of Unit L1 is a positive, timeline for its eventual clearance remains key, which analysts believe is at least a few quarters away. Investors should thereby keep a watch on developments from here on. Not surprisingly, the stock after gaining on Thursday gave up the gain and was down over five per cent in afternoon trades on Friday.
The early inspection at the facility comes as a positive surprise for the Street. Analysts at Macquarie say they have not built opening up of L1 till FY17-end. If the company is able to resolve regulatory issues at this facility by FY16-end, it would be a big booster to FY17 earnings.
Earlier, the company had offered all its facilities for inspection by the USFDA post-implementation of the desired GMP (good manufacturing practices) remediation in October 2014.
Investor confidence in Wockhardt, however, has been building up for some time after the Chikalthana plant getting approval for supplies to the UK from UK regulatory authority (UKMHRA) in December 2014, with the stock having almost doubled.
The UK revenues that were at £81 million (more that Rs 800 crore) in FY14 had already crossed £109 million (more than Rs 1,000 crore) during first nine months of FY15 and post-clearance, the UK business is to gain more traction. The company’s US-based Morton Grove facility, inspected by FDA earlier, had also not seen any adverse action by FDA (FDA accepted Wockhardt’s responses to their observations) and reports suggest Wockhardt has got clearance for the plant.
If the supplies to the US start sooner than expected, one can expect some earning upgrades.
If the company is able to start supplies after approval of newly expanded Shendra plant early in FY17, analysts at Macquarie arrive at FY17 EPS estimate of Rs 85 and target price of Rs 1,700 (without assuming any contribution from Waluj or Chikalthana).
But, if Chikalthana L1 unit gets approved by US FDA by end-FY16 or early FY17 and Wockhardt is able to recover 40-50 per cent of lost sales from L1 in FY17 (peak sales of $250 million) it could generate $100-125 million in incremental US sales resulting in FY17 EPS of around Rs 125 given the operating leverage that exist. And hence, the Macquarie analysts peg the target price at Rs 2,500.
Thus, while early inspection of Unit L1 is a positive, timeline for its eventual clearance remains key, which analysts believe is at least a few quarters away. Investors should thereby keep a watch on developments from here on. Not surprisingly, the stock after gaining on Thursday gave up the gain and was down over five per cent in afternoon trades on Friday.
More From This Section
Don't miss the most important news and views of the day. Get them on our Telegram channel
First Published: Mar 20 2015 | 10:35 PM IST
